Islet vascularized ECM gel (IVEG) constructs for transplantation into hypovascular sites and for studying islet-matrix-endothelial interactions
Daniel Tremmel1, Sara D Sackett1, Jon S Odorico1.
1Surgery - Division of Transplantation, University of Wisconsin, Madison, WI, United States
Introduction: Islet isolation results in extracellular matrix (ECM) damage, also severing connections with endothelial cells (ECs). Following transplantation, islets re-vascularize, enabling survival and function, but the time between isolation and revascularization results in significant islet loss. Further, much that is known about islet biology is based on research using isolated islets, which are missing crucial connections and signals from the native environment. We developed a culture system that reconstructs the islet ECM and creates a network of capillary-like endothelial tubes in vitro, prior to transplantation. The islet vascularized ECM gel (IVEG) enables the study of crosstalk between islets and the islet microenvironment in vitro.
Methods: Human pancreatic ECM hydrogel (hP-HG) was prepared by decellularization of pancreas, solubilization of the ECM, and neutralization to form a gel. Human ECs (HUVECs) were cultured in various islet, EC, and hybrid media to optimize 3-D tube formation (Fig 1A-B). Human islet function was assessed in IVEG constructs (Fig 2A) under ideal media conditions after 3 days of culture through static GSIS.
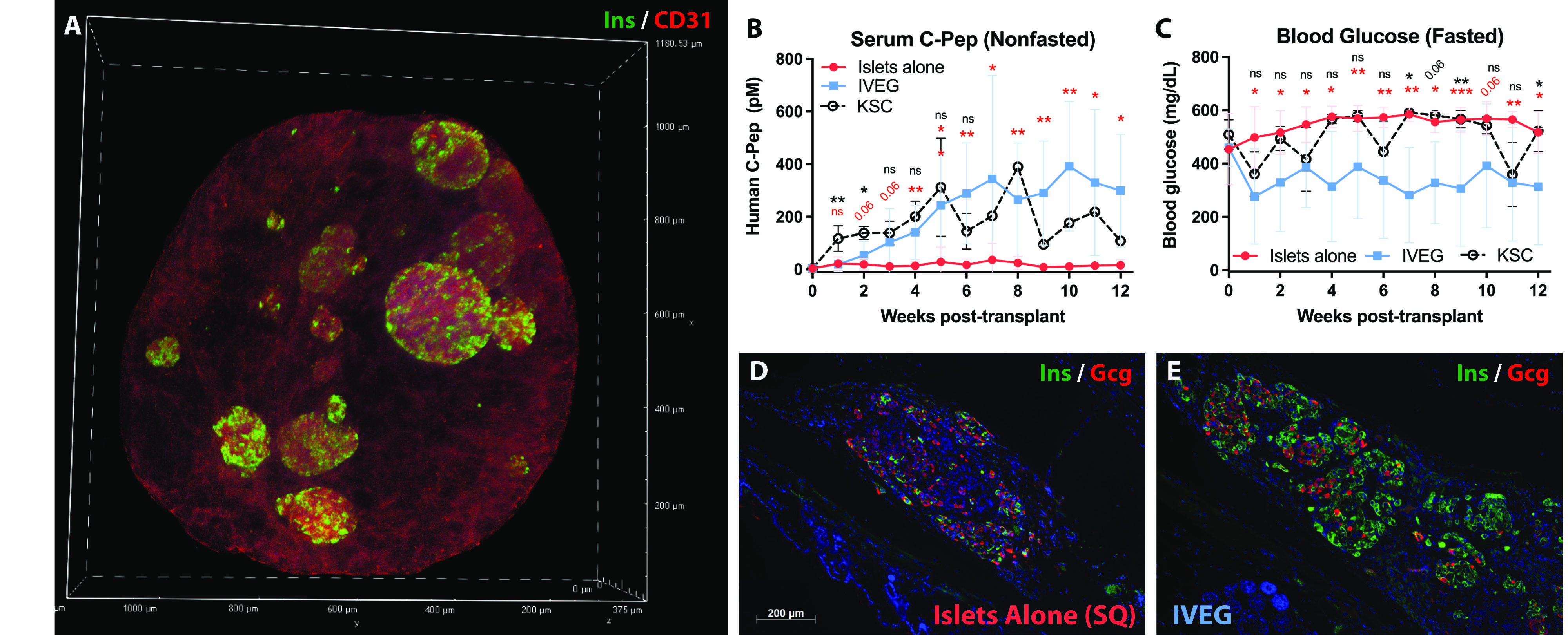
1000 IEQ were transplanted in diabetic immunodeficient mice (NSG RIP-DTR) alone subcutaneously (SQ), alone under the kidney subcapsule (KSC), or SQ within IVEG constructs.
Results: ECs cultured in hP-HG in EC growth medium (EGM) formed 3-D tubes covering 29% of the area of a max-intensity image (Fig 1C). In islet medium (CMRL), the percentage was reduced (14.2%) (Fig 1D), but when cultured in a hybrid medium combining EGM with CMRL, the coverage was 31.7% (Fig 1E). This methodology enables the co-culture of endothelial tubes with islets in hP-HG for several days (Fig. 2A). Islets cultured in the IVEG construct had a significantly improved stimulation index (SI = 10.0) compared to the same donor islets cultured in standard suspension culture (SI = 2.1). IVEG constructs were transplanted following 3 days of culture and EC network formation.
Diabetic mice did not experience stable serum insulin levels following SQ transplantation with 1000 IEQ alone, and did not have reductions in fasting blood glucose (BG) levels (Fig. 2B-C). Mice transplanted SQ with the same donor islets after 3 days of IVEG culture had reductions in fasted BG levels, and stable human insulin detected in serum (N=3 mice per group). 1000 IEQ transplanted SQ as an IVEG construct also outperformed 1000 IEQ from the same donors transplanted alone in the KSC. Grafts collected from the mice 12 weeks post-transplantation showed visibly better retention of beta cell mass in the IVEG groups (Fig. 2E) compared to SQ islets alone (Fig. 2D).
Conclusions: ECs are able to form 3-D tubes in hP-HG with maintained islet viability and function after 3 days of co-culture. This culture system enables the study of islet-endothelial cell interactions in vitro. Future studies will test how the IVEG constructs engraft and function after transplantation.